JADA
Access peer-reviewed research from The Journal of the American Dental Association, the premier dental journal since 1913.
Current issue
April 01, 2024
Featured
The authors present recommendations on radiation safety, appropriate imaging practices, and reducing radiation exposure.

April 01, 2024
The authors examine trends in data 1997-2019 data to identify progress and gaps in oral health disparities across sociodemographic groups.
April 01, 2024
The authors identify factors associated with oral hygiene compliance for 2 years after radiation therapy.
April 01, 2024
The authors review the evolution of the concept of sleep bruxism.
The latest articles from JADA, JADA Foundational Science and PracticeUpdate Clinical Dentistry Channel on LinkedIn and X, formerly known as Twitter.
Pieces from dental leaders addressing opinions about issues, events, and policies relevant to dentistry.
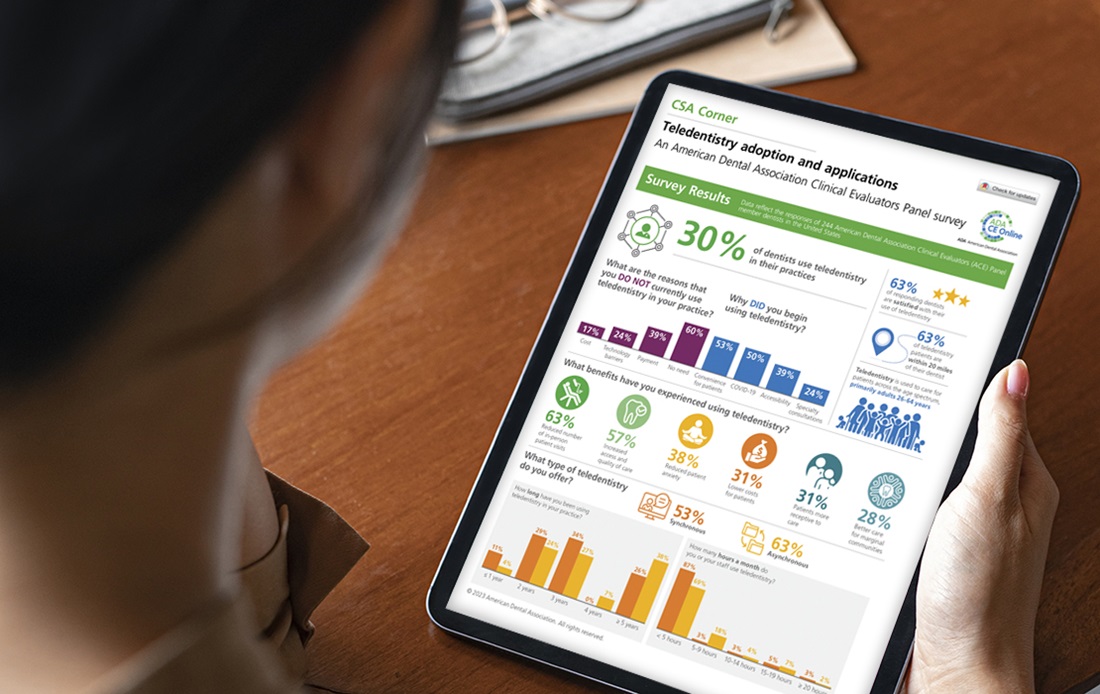
Teledentistry adoption and applications
Quick scan articles, CE offerings, and more to help you offer better care and improve oral health and well-being.
Explore all of JADA’s past issues, supplements and scans in the archives.