What is ANSI/ADA Standard No. 128 about?
This standard specifies the requirements and tests for helping determine whether elastic aqueous agar and alginate hydrocolloid dental impression materials are of the quality needed for their intended purposes. It also specifies requirements for labeling and instructions for use.
Download the Executive Summary (Free for ADA members)
Standard No. 128
What are the requirements I should know about for hydrocolloid impression materials?
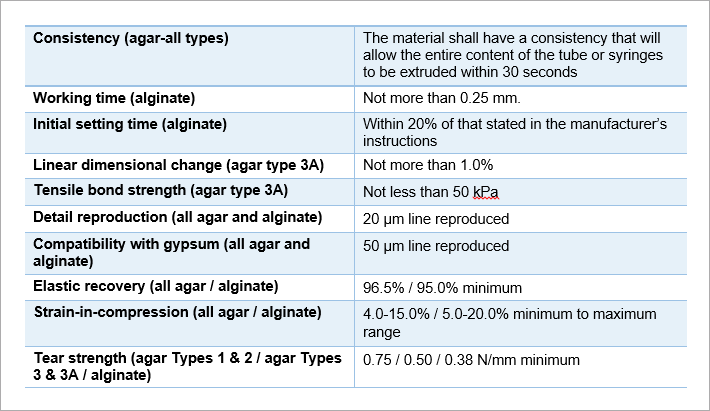
ANSI/ADA Standard No. 128 sets the following requirements to ensure the safety of the impression materials for use by consumers. The agar impression materials are classified into three types according to the consistencies they exhibit when ready for impressing against the oral or craniofacial tissue surfaces:
- Type 1: Heavy bodied, for making impressions of complete or partial dental arches, with or without the use of companion increments of lighter bodied Type 2 or Type 3 agar impression materials
- Type 2: Medium bodied, for making impressions of complete and partial dental arches, with or without the use of companion syringe-extruded increments of Type 3 agar materials
- Type 3: Light bodied, for syringe use with either the Type 1 or Type 2 agar materials
- Type 3A: Light bodied, material formulated for syringe use in a reversible/non-reversible impression material system, and that has been claimed to be capable of bonding to a companion alginate impression material that will make up the greater part of an agar/alginate impression material system
What’s the bottom line?
ANSI/ADA Standard No. 128 sets the requirements for consistency, working time, setting time, dimensional change, tensile bond strengths, detail reproduction, compatibility with gypsum, elastic recovery, strain-in-compression and tear strength for either agar or alginate impression materials that must be met for them to be safe for consumer use.