Last Updated: September 19, 2023
Anesthesia and Sedation
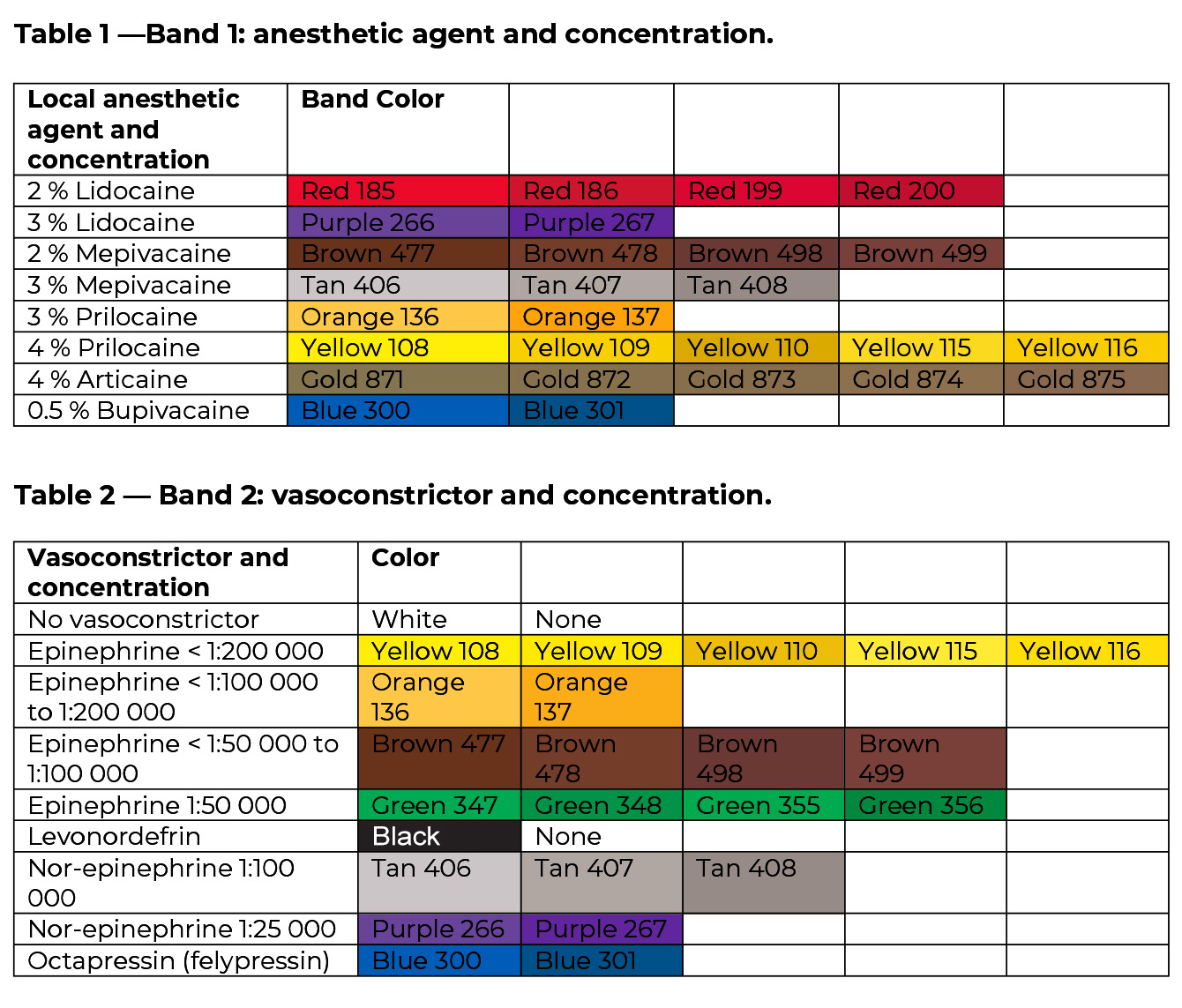
This color-coding system, adopted by the ADA Council on Scientific Affairs in 2003, has been incorporated into ANSI/ADA Standard No. 190:2020 for Single-use dental cartridges for local anesthetics (identical adoption of ISO standard 11499 Dentistry – Single-use cartridges for local anesthetics). Each single-use cartridge should have two indelible bands indicating the name and concentration of both the anesthetic ingredient (Table 1) and the vasoconstrictor (Table 2).
The tables below, which utilize the Pantone Matching System (PMS) for color identification, illustrate the appropriate colors for both ingredients. Although color-coding is a useful adjunct for anesthetic identification, it is prudent for dentists to read the labels of all drugs they administer.
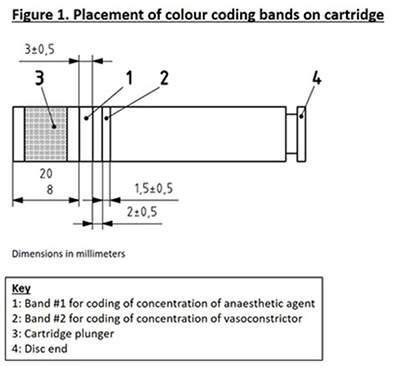
ANSI/ADA Standard No. 190:2020 (ISO standard 11499) outlines additional criteria required of single-use cartridge labels applicable to size and placement of the colored bands:
- Band 1 shall be 3.0 ± 0.5 mm wide and commence between 8 mm and 20 mm from the plunger end of the cartridge.
- Band 2 shall be 1.5 ± 0.5 mm wide and commence at (2 ± 0.5) mm from the cartridge disc end of Band 1.
A detailed diagram of a single-use cartridge can be found in Figure 1.
Dental Anesthesiology is a recognized dental specialty per the National Commission on Recognition of Dental Specialties and Certifying Boards. The recognition was awarded in March 2019 after the proposed specialty showed that it is distinct, well-defined, and requires unique and advanced knowledge and skills; and that it contributes new knowledge, education and research to the dental profession.
Guidelines for the Use of Sedation and General Anesthesia by Dentists (2016). Download a copy of the Guidelines (PDF).
Guidelines for Teaching Pain Control and Sedation to Dentists and Dental Students (2016). Download a copy of the Guidelines (PDF).
Guidelines for Teaching Pediatric Pain Control and Sedation to Dentists and Dental Students (2021). Download a copy of the Guidelines (PDF).
ADA Policy Statement: The Use of Sedation and General Anesthesia by Dentists (2007). Download a copy of the Policy Statement (PDF).
American Academy of Pediatric Dentistry:
- Preparing for Your Child’s Sedation Visit
- Policy for Selecting Anesthesia Providers for the Delivery of Office-based Deep Sedation/General Anesthesia
- Use of Anesthesia Providers in the Administration of Office-based Deep Sedation/General Anesthesia to the Pediatric Dental Patient
- Use of Local Anesthesia for Pediatric Dental Patients
- Use of Nitrous Oxide for Pediatric Dental Patients
American Academy of Pediatrics, American Academy of Pediatric Dentistry: Guidelines for Monitoring and Management of Pediatric Patients Before, During, and After Sedation
American Academy of Anesthesiologists: Practice Guidelines for Moderate Procedural Sedation and Analgesia 2018
Prepared by:
Research Services and Scientific Information, ADA Library & Archives.
Disclaimer
Content on the Oral Health Topics section of ADA.org is for informational purposes only. Content is neither intended to nor does it establish a standard of care or the official policy or position of the ADA; and is not a substitute for professional judgment, advice, diagnosis, or treatment. ADA is not responsible for information on external websites linked to this website.