Medication Safety Labeling
Historically, manufacturers have relied on an alphabetical system to communicate the safety of medications for use with pregnant patients. In 2015, the U.S. Food & Drug Administration began phasing out that system for prescription drugs, replacing it with a narrative section in the package insert that discusses the benefits and risks of using a particular medication with this population.9, 10 The new system will be phased-in, with a full compliance date of 2020.
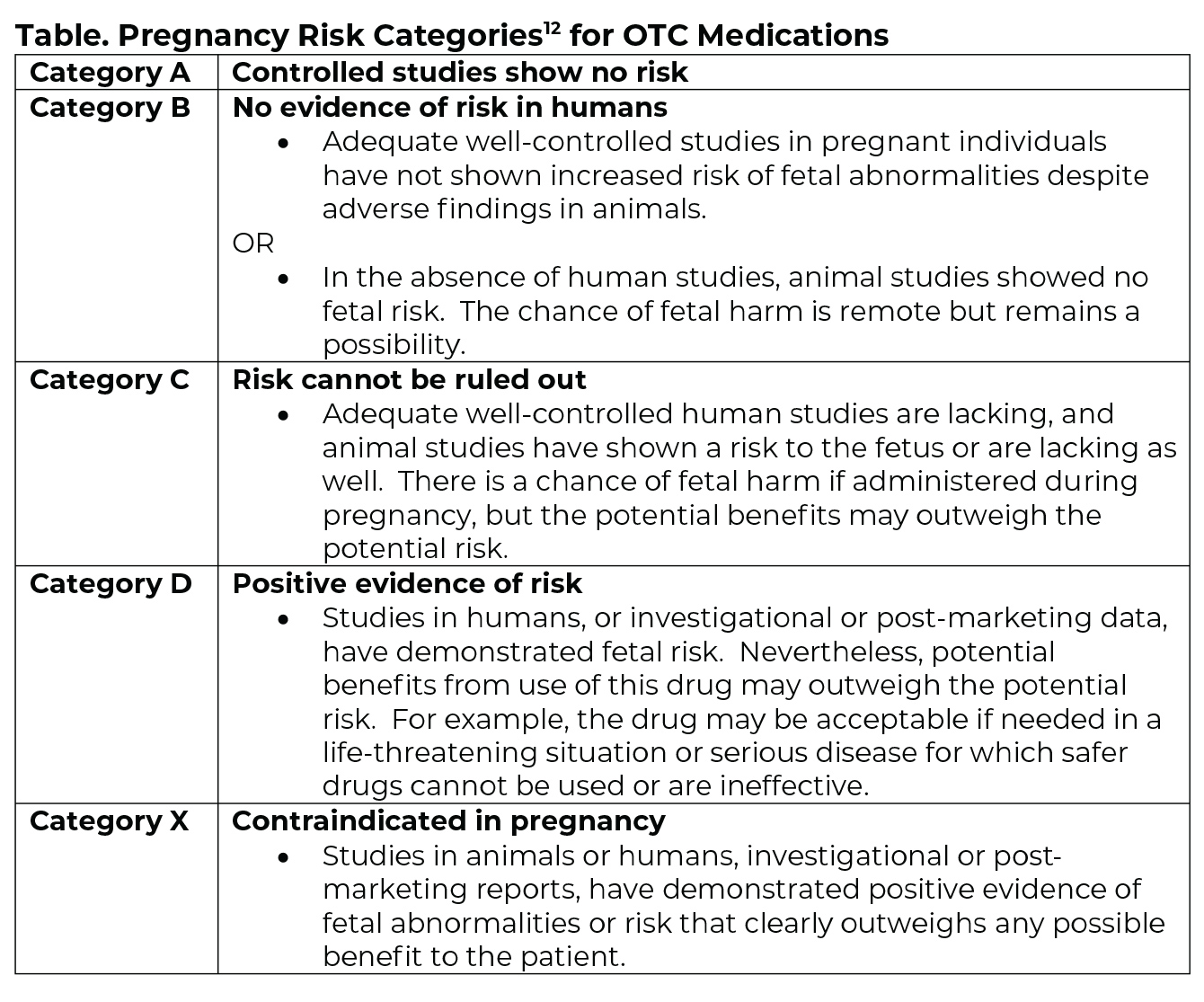
The alphabetical system (Table) will continue to be used for over-the-counter (OTC) medications.11
Medication Selection
Questions about use of local anesthetics or antibiotics in pregnant individuals are common. Options considered safe for use in these situations include:
- Local anesthesia (with or without epinephrine)1, 13-15
- Antibiotics13, 14, 16
- Penicillin
- Amoxicillin
- Cephalosporins
- Clindamycin
- Metronidazole
Use of other medications calls for consultation with the patient’s obstetrician to weigh risks and benefits. An example of a situation that may benefit from consultation is pain relief. Several analgesics had been placed in pregnancy Category B, which indicated that they were typically safe to use; however, in 2015, the U.S. Food & Drug Administration clarified that position, stating that the published research is “too limited to make any recommendations” on pain reliever use in this population.17 This suggests that decisions made about medications for pain relief should be arrived at after consultation with the obstetrician. That said, emergencies call for immediate implementation of standard emergency protocols.
Lactation
Questions often arise about medication use by patients who are lactating. Most medication product inserts have information related to use during lactation. The National Library of Medicine also provides a searchable database (LactMed) on this topic.
Nitrous Oxide
Nitrous oxide is classified as a pregnancy risk group Category C medication, meaning that there is a risk of fetal harm if administered during pregnancy. It is recommended that pregnant individuals, both patients and staff, avoid exposure to nitrous oxide.18 The National Institute of Occupational Safety and Health (NIOSH), a federal agency affiliated with the Centers for Disease Control and Prevention, recommends use of a scavenging system and exposure limits of N2O concentrations in dental operations to approximately 25 ppm during analgesia administration.19
Dental offices that use nitrous oxide-oxygen can review best management practices on the Nitrous Oxide Oral Health Topic page.
