- Roughly 42 percent of all dentate U.S. adults 30 years of age or older have periodontitis
- Attachment and bone loss associated with periodontal disease are results of the body’s immune response to plaque biofilm and its metabolic byproducts.
- While associations between periodontitis and various systemic conditions and diseases have been suggested by research, evidence of causality is mixed and the strength of the evidence differs for various conditions.
- In 2017 the World Workshop on the Classification for Periodontal and Peri-Implant Diseases and Conditions established a classification system for periodontitis that involves staging (i.e., the severity and extent of the disease) and grading (i.e., the potential for disease progression and treatment outcome).
- The goal of periodontal treatment is to eliminate dysbiotic plaque biofilm from the tooth surface and to establish an environment that allows the maintenance of health. This treatment of periodontal disease can be non-surgical or surgical with the optimal treatment being based on individual patient, site, and systemic factors.
Periodontitis
Key Points
NOTE: In 2017, the American Academy of Periodontology (AAP) and the European Federation of Periodontology (EFP) convened panels of experts to develop a classification system for periodontal and peri-Implant diseases and conditions.1 In 2018, these panels published consensus reports that described periodontal diseases including: periodontal health, gingival diseases and conditions2; periodontitis3, 4; and other conditions affecting the periodontium3, 4; as well as a system describing for peri-implant diseases (peri-implant health, peri-implant mucositis, and peri-implantitis).5 This Oral Health Topic page will focus on classifications related to periodontitis.
Periodontal disease is a chronic infection that can result in the destruction of tooth-supporting structures (i.e., the gingiva, periodontal ligament, and/or alveolar bone) and eventual tooth loss.6
According to analysis of data from the National Health and Nutrition Examination Survey (NHANES) collected from 2009 to 2014, roughly 42% of dentate adults 30 years of age or older in the United States have some form of periodontitis (mild, moderate or severe).7 The prevalence of periodontitis increases with age; it is significantly more common in males than in females, and in non-Hispanic Blacks and Hispanics than non-Hispanic whites.7
Periodontitis is an inflammatory disease of bacterial etiology resulting in loss of periodontal tissue attachment and alveolar bone. 8 The host response to the bacterial challenge leads to clinical signs such as deep pockets, bleeding on probing, gingival recession, and tooth mobility, which can ultimately cause tooth loss.
In 2018, the American Academy of Periodontology and the European Federation of Periodontology published the World Workshop Classification System for Periodontal and Peri-Implant Diseases and Conditions, which established a new approach to diagnosing periodontal disease to replace the system developed in 1999. The current classification system was endorsed by the American Dental Association in 2021.
Evidence of the association of periodontitis with systemic conditions is mixed (see the related Oral Health Topic page, Oral/Systemic Health). Associations, though not causal relationships, with periodontitis have been suggested for several conditions:
- Cardiovascular diseases: Although a causal relationship has not been established, the presence of periodontal disease has been associated with various cardiovascular diseases including myocardial infarction,9 hypertension,10 and carotid atherosclerosis.11 While research indicates a positive relationship between periodontal treatment and a short-term relationship on surrogate outcomes associated with cardiovascular disease, these studies have significant limitations and lack focus on true cardiovascular disease outcomes.12
- Diabetes: Periodontal disease and diabetes are considered to have a bidirectional relationship: hyperglycemia has an effect on oral health and periodontitis has an effect on glycemic control. A 2018 systematic review by Graziani et al. concluded that periodontitis is associated with (1) higher HbA1c levels in individuals without diabetes and in individuals with type 2 diabetes, (2) worsened diabetes-related complications in individuals with type 2 diabetes, and (3) an increased prevalence of complications in individuals with type 1 diabetes. The effect of periodontal treatment on diabetes-related parameters such as glycemic control is still inconclusive.13
- Respiratory diseases: Research suggests associations between periodontitis and respiratory diseases such as asthma, chronic obstructive pulmonary disease and pneumonia, possibly due to inflammatory processes and aspiration of microorganisms from the periodontal pocket. 14
- Pregnancy complications: An umbrella review of 23 systematic reviews found that periodontitis during pregnancy seems to contribute to increased risk of preterm birth, low birthweight infants and preeclampsia.15 However, there was no effect of periodontal treatment found on pre-term birth prevention.
- Rheumatoid arthritis: A 2020 systematic review indicates that periodontitis may increase the risk of developing rheumatoid arthritis.16
- Chronic kidney disease: Although there is no evidence on causal association, studies have shown a high periodontitis prevalence in chronic kidney disease populations also demonstrating racial and ethnic disparities.17
- Cancers: Periodontal disease and periodontal pathogens have been associated with cancers. 18
- Dementias: Periodontitis and periodontal pathogens have been associated with dementias and Alzheimer’s Disease. 19
Notably, the 2017 system published by AAP/EFP eliminates use of the diagnostic categories “Chronic” and “Aggressive” periodontitis. These are now considered under the general category of “periodontitis,” owing to the determination that extent and severity does not distinguish these as separate disease.3 Periodontitis is categorized by signs and symptoms of inflammation and attachment/radiographic bone loss.
Under the 2017 system, the current categories of periodontitis are3
- Necrotizing periodontal diseases
- Periodontitis
- Periodontitis as a Manifestation of Systemic Diseases
Necrotizing periodontal diseases feature papilla necrosis, bleeding, and pain, and are associated with impaired immune response,3 Periodontitis as a manifestation of systemic disease focuses on diseases and conditions other than diabetes (e.g., genetic disorders, neoplasms, and other metabolic and endocrine disorders).4, 20
Disease that does not meet these criteria are considered as periodontitis.
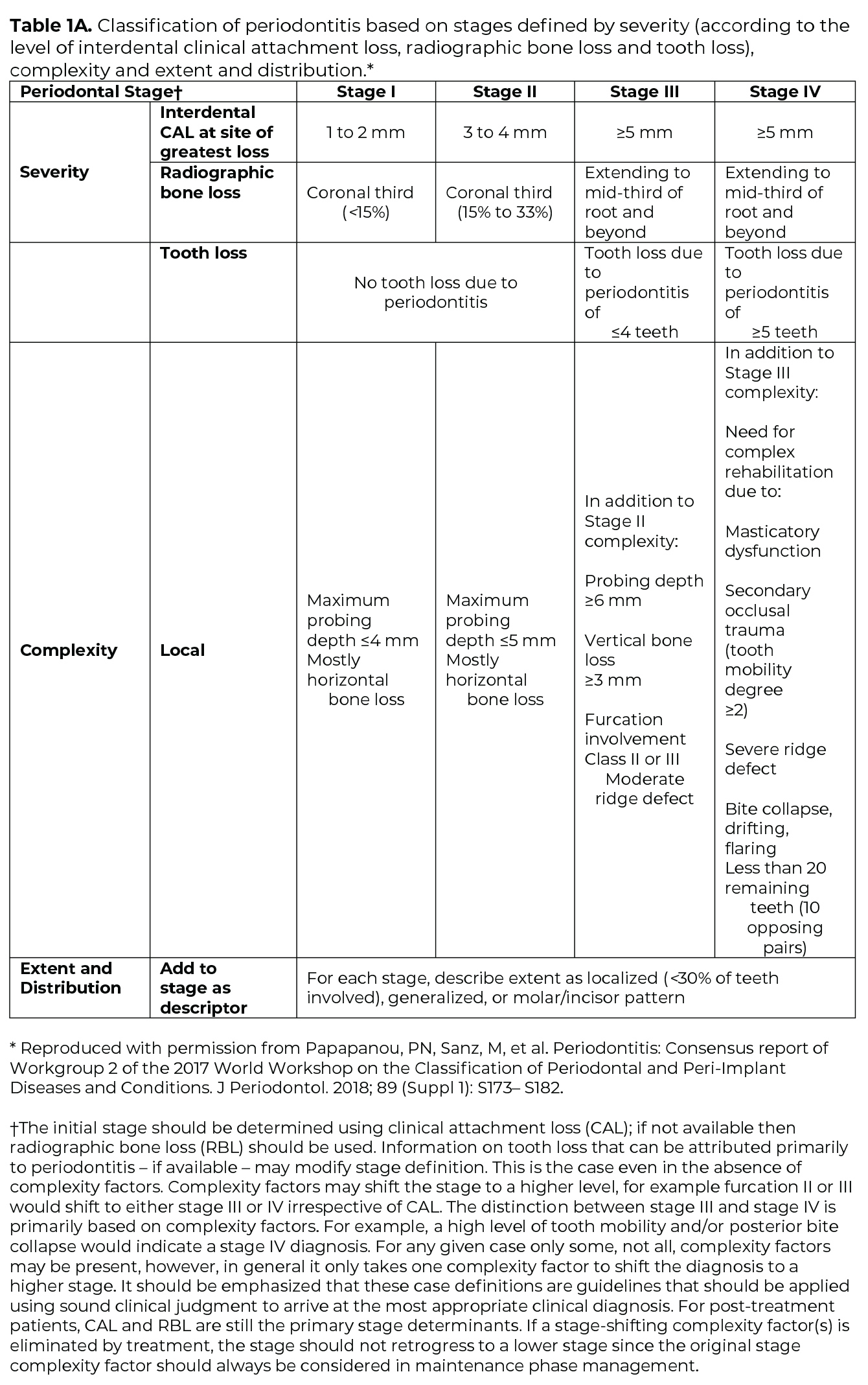
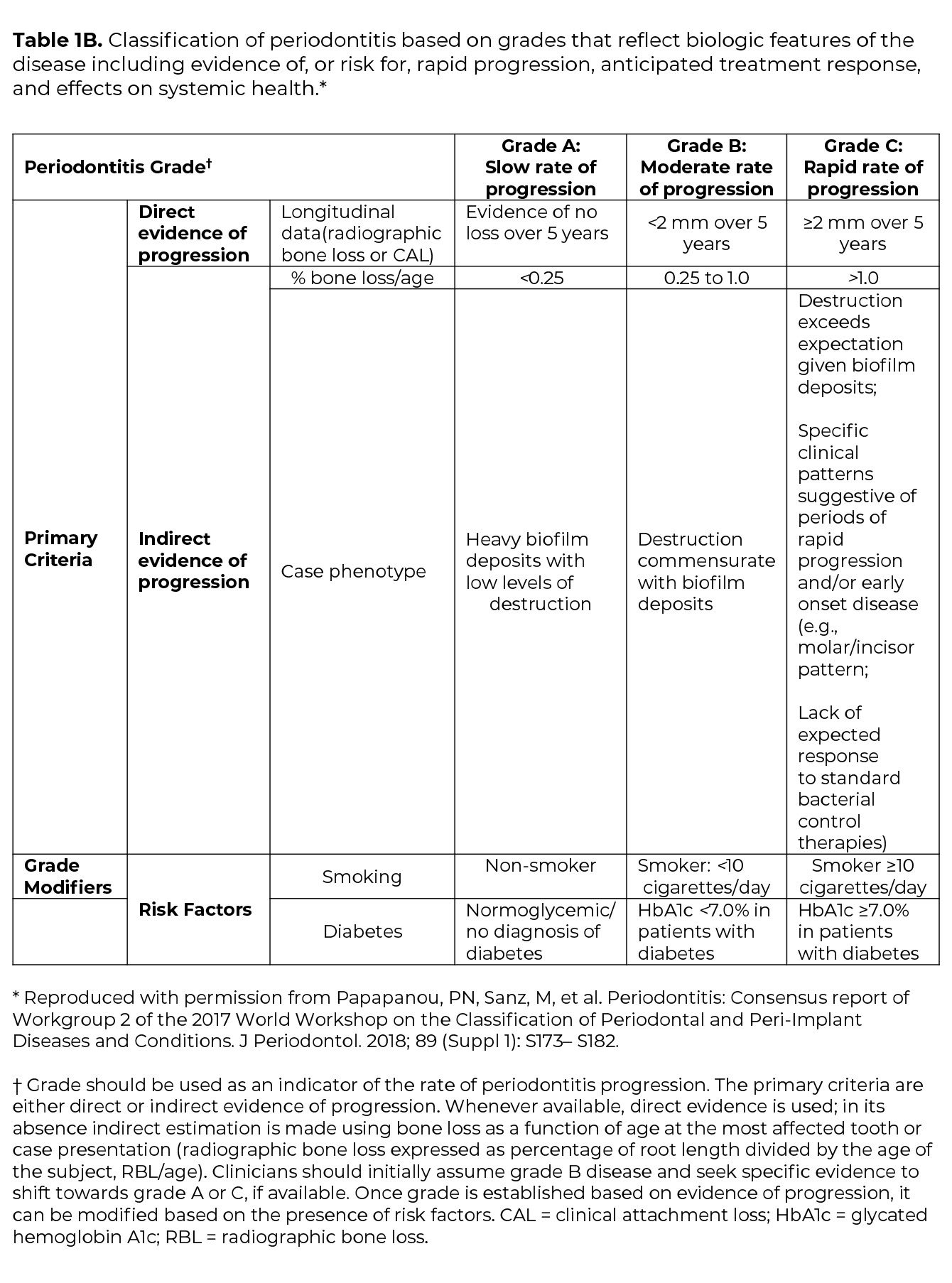
Once the determination of periodontitis has been made, the disease is classified according to one of four Stages (I-IV) based upon the most severe area of disease presentation, which describe the disease severity and extent of disease, focusing on attachment and bone loss (Table 1A). The extent of disease is categorized by the extent of stage-defining destruction. After the Stage is determined, the case is assigned one of three Grades (A, B, C) that indicate the potential for disease progression and treatment outcome (Table 1B). Grading is based on supplemental considerations like direct evidence of disease progression, indirect evidence of disease progression (radiographic bone loss divided by age), smoking patterns, and diabetes and glycemic control.3
The goal of periodontal treatment is to eliminate plaque, biofilm and calculus, from the tooth surface and establish an environment that can be maintained in health.21 Treatment of periodontitis can be non-surgical or surgical. The optimal treatment is based on the patient, site and systemic factors.
Non-Surgical
The American Academy of Periodontology defines non-surgical treatment as the professional removal of supragingival and subgingival bacterial plaque or biofilm and calculus, which provides a biologically acceptable root surface, as well as patient adoption of a comprehensive daily plaque or biofilm control routine. 22
Non-surgical therapy includes21:
- Patient education and oral hygiene instruction
- Complete removal of supragingival calculus
- Restoration or temporization of carious lesions
- Treatment of areas where plaque and food debris can collect, including orthodontic treatment and removal of plaque retentive factors.
According to ADA clinical practice guidelines on non-surgical treatment,23 derived from a 2015 systematic review,23 scaling and root planing without adjuncts is the treatment of choice for patients who have periodontitis. The guidelines go on to endorse use of systemic sub-antimicrobial dose doxycycline along with scaling and root planing for patients with moderate-to-severe periodontitis. Specifically, the guidelines recommend oral doxycycline (20 mg twice a day) for 3 to 9 months following scaling and root planing for these patients.
Patients often require several treatment sessions for complete debridement of the tooth surfaces.21 After scaling, root planing, and other adjunctive treatment approaches such as use of antibiotic therapy, the periodontal tissues require approximately 4 weeks to demonstrate optimal effects of nonsurgical therapy.21
Surgical
Many moderate to advanced cases require surgical access to the root surface for root planing and reducing pocket depth, which will allow the patient to achieve successful home care.21
Surgical treatment entails21:
- Correction of anatomic conditions that predispose the patient to periodontitis, impair aesthetics, or impede placement of prosthetic appliances
- Extraction of teeth that cannot be successfully treated
- Placement of implants when teeth are lost
Surgical treatment options include24:
- Gum Graft Surgery: Gum graft surgery is intended to prevent further gingival (gum) recession and bone loss and to reduce sensitivity. During this procedure, tissue is taken from the palate or another donor source to cover exposed roots.
- Periodontal Pocket Reduction Procedures: In this approach, gingival tissue is folded back to allow for removal of disease-causing bacteria, after which the tissue is sutured back in place. This is intended to allow gingival tissue to reattach to the bone.
- Regenerative Procedures: These are procedures that are performed when there is bone destruction. Once again, the gingival tissue is folded back and the disease-causing bacteria are removed, after which membranes, bone grafts, or tissue-stimulating proteins are used to help promote regeneration of supporting periodontal tissues.
Maintenance
A patient with gingivitis can revert to a state of health with a reduced periodontium, but due to the host-related disease susceptibility, a periodontitis patient remains a periodontitis patient, even following successful therapy, and requires life‐long supportive care to prevent recurrence of disease.2 Further, patients with more severe periodontitis Stage and Grade have been found to be more likely to experience disease recurrence and tooth loss without regular periodontal maintenance visits. 25
Much of the literature agrees that, after non-surgical and/or surgical periodontal treatment, patients could benefit from more frequent visits, possibly every 3-6 months.26, 27 These appointments could include a review of home oral hygiene behaviors, ascertainment of exposure to risk factors such as tobacco use, professional plaque removal, and subgingival debridement, as needed.26-28 Patients also could be assessed to determine if active therapy is needed to treat recurrent periodontal disease.27
Researchers generally agree the maintenance phase is key to allow for close monitoring of the attachment level and pocket depth along with the other clinical variables, such as bleeding, exudation, tooth mobility.21
- Caton JG, Armitage G, Berglundh T, et al. A new classification scheme for periodontal and peri-implant diseases and conditions - Introduction and key changes from the 1999 classification. J Clin Periodontol 2018;45 Suppl 20:S1-S8.
- Chapple ILC, Mealey BL, Van Dyke TE, et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 2018;89 Suppl 1:S74-S84.
- Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol 2018;45 Suppl 20:S162-S70.
- Jepsen S, Caton JG, Albandar JM, et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol 2018;45 Suppl 20:S219-S29.
- Berglundh T, Armitage G, Araujo MG, et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol 2018;45 Suppl 20:S286-S91.
- Periodontal Disease and Overall Health: A Clinician’s Guide. Yardley, PA: Professional Audience Communications, Inc.; 2010.
- Eke PI, Borgnakke WS, Genco RJ. Recent epidemiologic trends in periodontitis in the USA. Periodontol 2000 2020;82(1):257-67.
- Genco R, Williams R. Periodontal Disease and Overall Health: A Clinician’s Guide. Yardley, PA: Professional Audience Communications, Inc.; 2010.
- Xu S, Song M, Xiong Y, et al. The association between periodontal disease and the risk of myocardial infarction: a pooled analysis of observational studies. BMC Cardiovasc Disord 2017;17(1):50.
- Martin-Cabezas R, Seelam N, Petit C, et al. Association between periodontitis and arterial hypertension: A systematic review and meta-analysis. Am Heart J 2016;180:98-112.
- Zeng XT, Leng WD, Lam YY, et al. Periodontal disease and carotid atherosclerosis: A meta-analysis of 17,330 participants. Int J Cardiol 2016;203:1044-51.
Roca-Millan E, Gonzalez-Navarro B, Sabater-Recolons MM, et al. Periodontal treatment on patients with cardiovascular disease: Systematic review and meta-analysis. Med Oral Patol Oral - Cir Bucal 2018;23(6):e681-e90.
- Cao R, Li Q, Wu Q, et al. Effect of non-surgical periodontal therapy on glycemic control of type 2 diabetes mellitus: a systematic review and Bayesian network meta-analysis. BMC Oral Health 2019;19(1):176.
- Gomes-Filho IS, Cruz SSD, Trindade SC, et al. Periodontitis and respiratory diseases: A systematic review with meta-analysis. Oral Dis 2020;26(2):439-46.
- Daalderop LA, Wieland BV, Tomsin K, et al. Periodontal Disease and Pregnancy Outcomes: Overview of Systematic Reviews. JDR Clin Trans Res 2018;3(1):10-27.
- Qiao Y, Wang Z, Li Y, et al. Rheumatoid arthritis risk in periodontitis patients: A systematic review and meta-analysis. Joint Bone Spine 2020;87(6):556-64.
- Ioannidou E, Hall Y, Swede H, Himmelfarb J. Periodontitis associated with chronic kidney disease among Mexican Americans. J Public Health Dent 2013;73(2):112-9.
- Nwizu N, Wactawski-Wende J, Genco RJ. Periodontal disease and cancer: Epidemiologic studies and possible mechanisms. Periodontol 2000 2020;83(1):213-33.
- Ma KS, Hasturk H, Carreras I, et al. Dementia and the Risk of Periodontitis: A Population-Based Cohort Study. J Dent Res 2022;101(3):270-77.
- Albandar JM, Susin C, Hughes FJ. Manifestations of systemic diseases and conditions that affect the periodontal attachment apparatus: Case definitions and diagnostic considerations. J Clin Periodontol 2018;45 Suppl 20:S171-S89.
- Newman MG, Takei HH. Newman and Carranza’s Clinical Periodontology. 13th ed. ed. Philadelphia: Elsevier; 2019.
- American Academy of Periodontology. Ad Hoc Committee on the Parameters of Care: Phase I therapy. J Periodontol 2000;71(Supplement):856.
- Smiley CJ, Tracy SL, Abt E, et al. Evidence-based clinical practice guideline on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc 2015;146(7):525-35.
- American Academy of Periodontology Surgical Procedures. "https://www.perio.org/for-patients/periodontal-treatments-and-procedures/surgical-procedures/ ". Accessed March 7 2020.
- Ravida A, Galli M, Saleh MHA, et al. Maintenance visit regularity has a different impact on periodontitis-related tooth loss depending on patient staging and grading. J Clin Periodontol 2021;48(8):1008-18.
- Leow NM, Moreno F, Marletta D, et al. Recurrence and progression of periodontitis and methods of management in long-term care: A systematic review and meta-analysis. J Clin Periodontol 2021.
- Kwon T, Lamster IB, Levin L. Current Concepts in the Management of Periodontitis. Int Dent J 2021;71(6):462-76.
- Manresa C, Sanz-Miralles EC, Twigg J, Bravo M. Supportive periodontal therapy (SPT) for maintaining the dentition in adults treated for periodontitis. Cochrane Database Syst Rev 2018;1:CD009376.
Professional Resources
- Search JADA for articles related to periodontitis
- Clinical Practice Guideline for Nonsurgical Treatment of Chronic Periodontitis
ADA Oral Health Topic Pages:
o Diabetes
o Oral-Systemic Health
o Pregnancy - ADA Library Services
- ADA Store
o ADA Flip Guide to Periodontal Disease (W463)
o Periodontal Disease: Keep Your Gums Healthy (W107)
o Periodontal Disease: Don’t Wait Until it Hurts (W12120)
o Periodontal Disease: Your Complete Guide (W120)
o Healthy Mouth, Healthy Body: Making the Connection (W203)
Patient Resources
- For the Patient: Keeping an Eye on Your Gums
- MouthHealthy: Gum disease
Last Updated: June 9, 2022
Prepared by:
Research Services and Scientific Information, ADA Library & Archives.
Disclaimer
Content on the Oral Health Topics section of ADA.org is for informational purposes only. Content is neither intended to nor does it establish a standard of care or the official policy or position of the ADA; and is not a substitute for professional judgment, advice, diagnosis, or treatment. ADA is not responsible for information on external websites linked to this website.