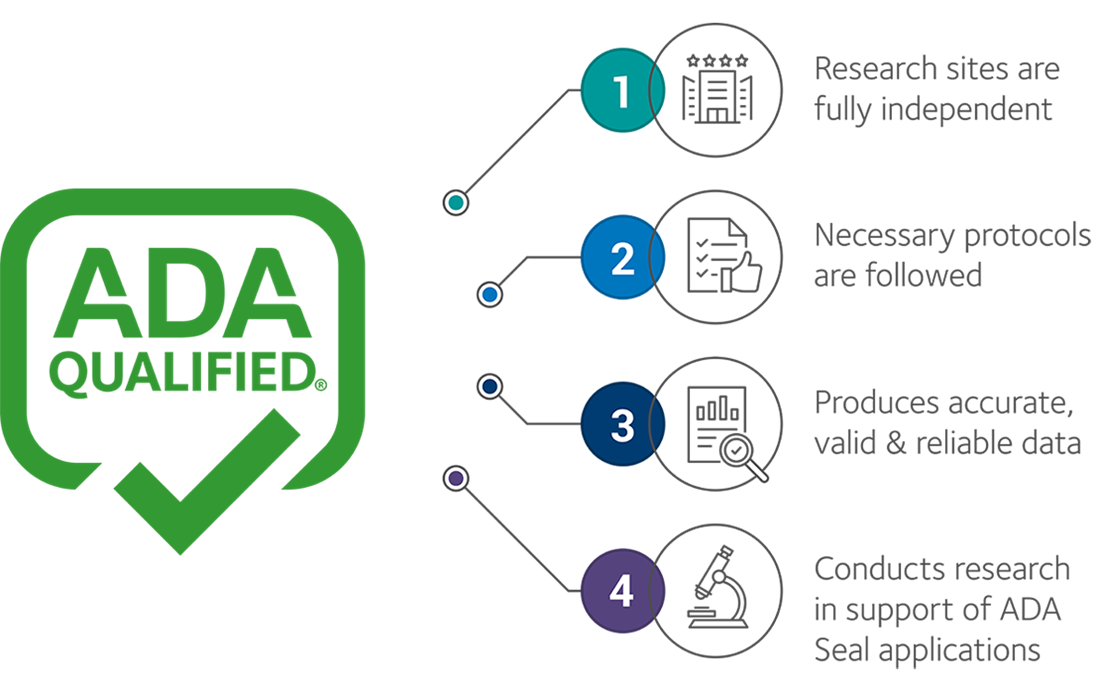
The ADA Qualified Sites program connects manufacturers of OTC oral health care products with research sites qualified to test products’ safety and efficacy. Manufacturers applying to the ADA Seal program may choose from the ADA Qualified Sites directory of vetted research sites, ensuring high quality studies are conducted without the need for additional confirmatory testing.
If the site meets these strict criteria, it earns Qualified status. This indicates to product manufacturers that the ADA has evaluated the site for its ability to conduct quality research in support of Seal Program applications. By ensuring the research sites are fully independent, follow all the necessary protocols and produce accurate, valid and reliable data, the ADA Seal program receives the best non-biased data with which to evaluate product submissions.