BPA is widely used in the production of polycarbonate plastics, polyacrylate resins, and epoxy resins.7 Although BPA is not used in the manufacture of dental materials, it is used in the synthesis of monomers common to dental resins, such as Bis-GMA, Bis-EMA, Bis-DMA, and BADGE (please see abbreviation list at the end). Residual BPA may remain from the synthesis process of these monomers and thus trace amounts may be present as a contaminant in dental resins.9, 17,18 Bis-DMA is a monomer occasionally used, in small amounts, to decrease viscosity of dental sealants. It is the only monomer that when exposed to salivary esterase may break down to form BPA.4
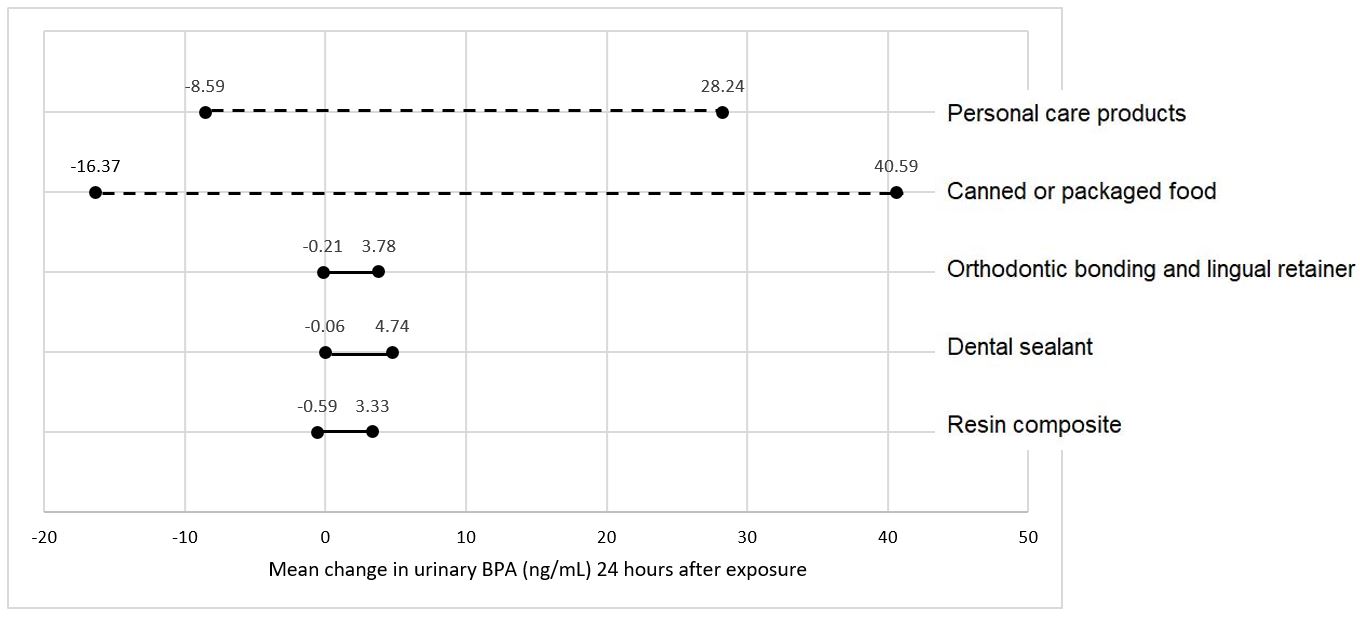
Trace amounts of residual BPA may leach out of the freshly polymerized resin and contribute to a small, transient (Figure 1) increase in BPA levels in saliva and urine following procedures involving resin-based dental materials.4, 19-21 Wear of the soft, underpolymerized surface layer of unpolished composite restorations is likely the reason for the clinical observation that the majority of BPA released in saliva occurs within 24-48 hours post-placement.20-22 Inadequate curing and exposing resin based materials to saliva may further exacerbate BPA exposure.4, 15
BPA in Dental Sealants
Placement of resin based dental sealants can result in small, transient increase in BPA detected in children’s saliva and urine (Figure 1).19, 21 The magnitude of the change, which typically resolves within 24-48 hours after dental sealant placement, is dependent on the number of teeth treated with sealant and/or resin composite.22 The main source of BPA is believed to be the outer layer of sealants and resin composites that does not polymerize in the presence of oxygen.19 Washing the unpolymerized resin layer away, and/or asking patients to rinse their mouths, may help reduce potentially free BPA leaching following sealant placement.19
BPA in Resin Composite
Research indicates that there is a transient increase in the amount of BPA measured following placement of composite resin restorations (Figure 1).21 Longer follow-up studies showed salivary or urinary BPA levels back to pre-treatment levels after 2-4 weeks following dental procedures involving sealant or composite restorations.20, 22, 23 Leaching of unreacted monomers is linked to polymerization condition and poor degree of conversion of resin composites.19, 20
BPA in Orthodontic Bonding Materials
Resin-based orthodontic adhesives19 used to bond brackets to teeth surfaces are also composed of BPA derivatives, such as Bis-GMA and Bis-EMA. Temporary increase of BPA content in saliva has been observed after bracket bonding (Figure 1),20 likely due to the presence of an oxygen-inhibited layer after light-curing of the orthodontic adhesive, which is exposed to saliva. Asking patients to rinse their mouths immediately after bonding of orthodontic devices helps minimize ingestion.2
Figure 1. Range of BPA detection in urine by different sources of exposure.11, 19-21, 24, 25